
Molecules. Atoms glued by bonds; nuclei incarcerated by electrons; electrons forming an inhomogeneous gas contained not by outer walls but by an electrostatic potential in its interior ironically named ‘external potential’. Molecules. The study object of chemists. The fundamental construct on which the chemical understanding of the universe relies.
Ten electrons, ten protons, and ten neutrons, giving rise to various electronic densities, various chemical properties: CH4, NH3, H2O, HF; which is it?
Atoms are letters, molecules are words; Chemistry, their unabashed poetry.

Canonical Molecular Orbitals are–by construction–delocalized over the various atoms making up a molecule. In some contexts it is important to know how much of any given orbital is made up by a particular atom or group of atoms, and while you could calculate it by hand given the coefficients of each MO in terms of every AO (or basis set function) centered on each atom there is a straightforward way to do it in Gaussian.
If we’re talking about ‘dividing’ a molecular orbital into atomic components, we’re most definitely talking about population analysis calculations, so we’ll resort to the pop keyword and the orbitals option in the standard syntax:
This will produce the following output right after the Mulliken population analysis section:
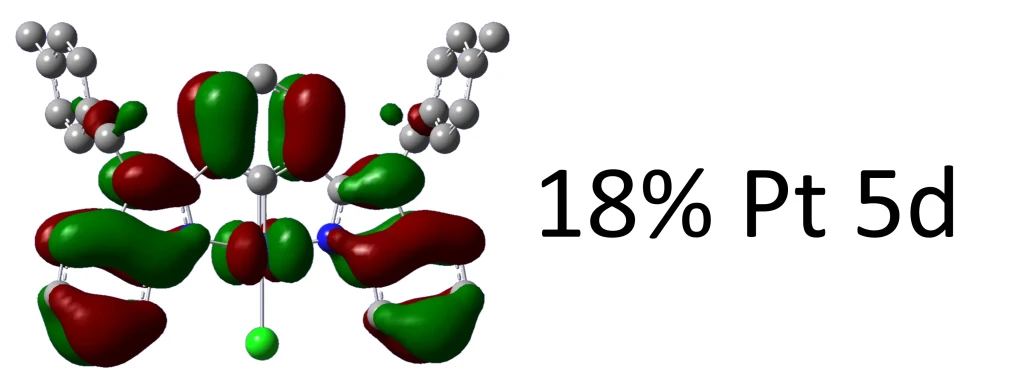
Atomic contributions to Alpha molecular orbitals: Alpha occ 140 OE=-0.314 is Pt1-d=0.23 C38-p=0.16 C31-p=0.16 C36-p=0.16 C33-p=0.15 Alpha occ 141 OE=-0.313 is Pt1-d=0.41 Alpha occ 142 OE=-0.308 is Cl2-p=0.25 Alpha occ 143 OE=-0.302 is Cl2-p=0.72 Pt1-d=0.18 Alpha occ 144 OE=-0.299 is Cl2-p=0.11 Alpha occ 145 OE=-0.298 is C65-p=0.11 C58-p=0.11 C35-p=0.11 C30-p=0.11 Alpha occ 146 OE=-0.293 is C58-p=0.10 Alpha occ 147 OE=-0.291 is C22-p=0.09 Alpha occ 148 OE=-0.273 is Pt1-d=0.18 C11-p=0.12 C7-p=0.11 Alpha occ 149 OE=-0.273 is Pt1-d=0.18 Alpha vir 150 OE=-0.042 is C9-p=0.18 C13-p=0.18 Alpha vir 151 OE=-0.028 is C7-p=0.25 C16-p=0.11 C44-p=0.11 Alpha vir 152 OE=0.017 is Pt1-p=0.10 Alpha vir 153 OE=0.021 is C36-p=0.15 C31-p=0.14 C63-p=0.12 C59-p=0.12 C38-p=0.11 C33-p=0.11 Alpha vir 154 OE=0.023 is C36-p=0.13 C31-p=0.13 C63-p=0.11 C59-p=0.11 Alpha vir 155 OE=0.027 is C65-p=0.11 C58-p=0.10 Alpha vir 156 OE=0.029 is C35-p=0.14 C30-p=0.14 C65-p=0.12 C58-p=0.11 Alpha vir 157 OE=0.032 is C52-p=0.09 Alpha vir 158 OE=0.040 is C50-p=0.14 C22-p=0.13 C45-p=0.12 C17-p=0.11 Alpha vir 159 OE=0.044 is C20-p=0.15 C48-p=0.14 C26-p=0.12 C54-p=0.11
Alpha and Beta densities are listed separately only in unrestricted calculations, otherwise only the first is printed. Each orbital is listed sequentially (occ = occupied; vir = virtual) with their energy value (OE = orbital energy) in atomic units following and then the fraction with which each atom contributes to each MO.
By default only the ten highest occupied orbitals and ten lowest virtual orbitals will be assessed, but the number of MOs to be analyzed can be modified with orbitals=N, if you want to have all orbitals analyzed then use the option All Orbitals instead of just orbitals. Also, the threshold used for printing the composition is set to 10% but it can be modified with the option Thresh Orbitals=N, for the same compound as before here’s the output lines for HOMO and LUMO (MOs 149, 150) with Thresh Orbitals set to N=1, i.e. 1% as occupation threshold (Thresh Orbitals=1):
Alpha occ 149 OE=-0.273 is Pt1-d=0.18 N4-p=0.08 N6-p=0.08 C20-p=0.06 C13-p=0.06 C48-p=0.06 C9-p=0.06 C24-p=0.05 C52-p=0.05 C16-p=0.04 C44-p=0.04 C8-p=0.03 C15-p=0.03 C17-p=0.03 C45-p=0.02 C46-p=0.02 C18-p=0.02 C26-p=0.02 C54-p=0.02 N5-p=0.01 N3-p=0.01
Alpha vir 150 OE=-0.042 is C9-p=0.18 C13-p=0.18 C44-p=0.08 C16-p=0.08 C15-p=0.06 C8-p=0.06 N6-p=0.04 N4-p=0.04 C52-p=0.04 C24-p=0.04 N5-p=0.03 N3-p=0.03 C46-p=0.03 C18-p=0.03 C48-p=0.02 C20-p=0.02



