In short, because it is a hard problem to solve. Our bodies are designed to prevent foreign DNA to make it into their cells and use its machinery to produce products. Normally, this is what pathogens do but it is the same route that gene therapy needs to traverse as well. Nonetheless, there has been some success in gene therapy especially in the past few years and the future looks promising in my view.
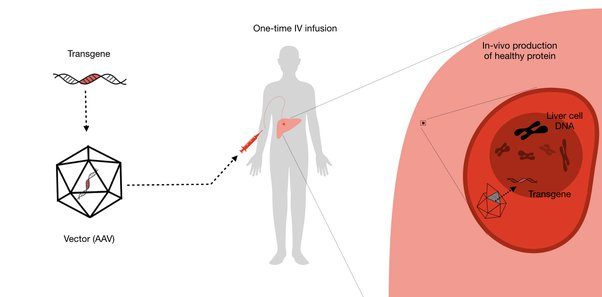
Gene therapy is an attempt to trick the body to accept foreign DNA, and let it make some product inside the body for a long period of time. For this, you need (1) a delivery mechanism that is both specific to target cells, non-toxic, and non-immunogenic (2) a good genetic construct that can produce the desired product in the right cells, and remain stable for a period of time. Even then you could only target diseases that can benefit from the action of a single gene.
My company, Dyno Therapeutics, works on the delivery problem, specifically we use the capsid protein of a harmless human virus known as Adeno-associated Virus (AAV). These are the basis for the any FDA approved gene therapies so far (e.g. Luxturna). AAVs have been studied for this purpose for two decades in different trials as delivery platforms. However they still demonstrate shortcomings. First, due to their natural prevalence, many people have pre-existing immunity to them which makes it hard to use as a vector (because the immune system clear it before it reaches the target tissue). Second, natural viruses are not specialized at targeting many tissues in the body. To make them reach the tissue of interest at a high enough dose, one would need to administer a lot of virus to the patient, which in a lot of cases can cause liver toxicity (because liver is a favorite destination for the virus, and it also has to clear the virus from circulation).
The truth is there is no broadly effective vector, and the field is still struggling to find vectors that are good at delivering to key organs like the brain. In our view at Dyno, the delivery problem is one of the key obstacles in making gene therapies a reality. We think that we can solve this problem by combining AI-driven design with high-throughput synthesis platforms. You can read about our approach’s philosophy in this post.
Gene therapy holds incredible promise but faces significant challenges, particularly in the realm of delivery mechanisms. One of the critical components in successful gene therapy is the vector—the carrier that transports therapeutic genes into target cells within the body. Current vectors, like Adeno-associated Viruses (AAVs), while promising, have limitations that hinder their widespread application.
AAVs, derived from a harmless human virus, have been the basis for many FDA-approved gene therapies such as Luxturna. However, their natural prevalence means that a significant portion of the population may already have antibodies against them, which can neutralize the virus before it can deliver its payload. This pre-existing immunity complicates the use of AAVs as effective vectors for gene therapy, especially in patients who have been exposed to these viruses naturally or through previous treatments.
Moreover, AAVs are not inherently specialized to target specific tissues or organs efficiently. This lack of specificity necessitates higher doses of the virus to ensure enough reaches the target tissue, which can lead to potential toxicity concerns, particularly in organs like the liver, which naturally attract and clear AAVs from circulation.
Addressing these challenges is crucial for advancing gene therapy to its full potential. Dyno Therapeutics, a company focused on solving the delivery problem, aims to overcome these limitations using innovative approaches. They propose combining AI-driven design with high-throughput synthesis platforms to develop improved AAV vectors.
The AI-driven design process at Dyno involves computational algorithms that can predict and optimize the properties of AAV capsid proteins. Capsid proteins are crucial because they determine the virus’s ability to evade the immune system, target specific cells, and deliver genetic payloads effectively. By using AI to analyze vast amounts of data, Dyno seeks to engineer AAV capsids that are less likely to be recognized by pre-existing antibodies and more efficient at targeting desired tissues.
Furthermore, Dyno employs high-throughput synthesis platforms to rapidly prototype and test these engineered capsids. This approach accelerates the discovery process, allowing for the screening of numerous capsid variants to identify those with the most desirable properties—such as reduced immunogenicity, enhanced tissue specificity, and improved payload delivery.
In essence, Dyno Therapeutics’ strategy represents a significant step forward in overcoming the longstanding challenges of gene therapy delivery. By leveraging cutting-edge technologies and computational approaches, they aim to develop next-generation AAV vectors that can effectively navigate the complexities of the human body, delivering therapeutic genes precisely where they are needed without triggering harmful immune responses or causing undue toxicity.
As the field continues to evolve, innovations from companies like Dyno hold the potential to expand the applicability of gene therapy beyond single-gene disorders to more complex diseases that require precise and effective genetic interventions. By addressing the delivery problem head-on, Dyno Therapeutics is contributing to the realization of gene therapy’s promise as a transformative medical technology.


